Indications for botulinum A toxin treatment in urology
- To treat Detrusor Overactivity associated incontinence refractory to medical treatment
- To treat voiding dysfunction – Detrusor Sphincter Dysynergia, dysfunctional voiding, detrusor underactivity
- To treat urgency syndrome – sensory urgency, chronic IC, low bladder compliance
- To treat BPH and chronic prostatitis
- To treat chronic pelvic pain syndrome
Introduction
Botulinum toxin was first isolated as a causative toxin of botulism in 1897 by van Ermengem by isolating the spore forming obligate anaerobic bacteria, Clostridium botulinum.
Immunologically 7 types designated as A,B,C,D,E,F & G and are the most potent naturally occuring toxins known to man.
Today the selective administration of therapeutic doses of botulinum toxin manufactured for medical use has become clinically valuable in many neuromuscular disorders .
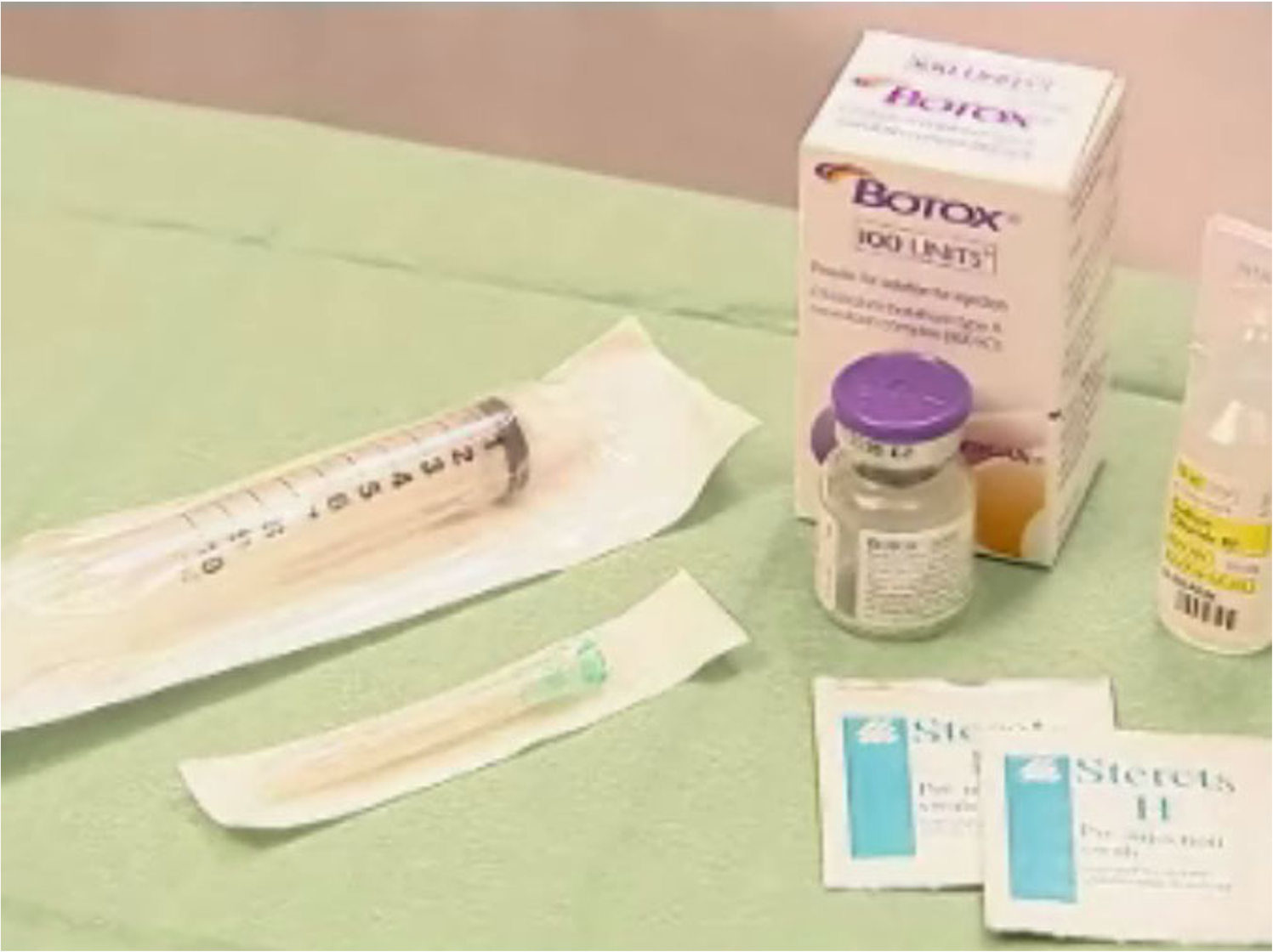

Vacuum dried powder stored in a refrigerator at or below 2C to8C 100 Units toxin diluted with preservative free NS Use within 4 hours
Commercial BTX
- 3 commercial forms are available worldwide
-
BTX-A
- BOTOX ( Allergan, Irvine, California)
- DYSPORT ( Ipsen Luxembourg)
-
BTX-B
- MYOBLOC (Elan, Dublin Ireland) (mainly used for plastic & ophthalmic purposes)
Commercial Preparations
- We have used Botox in our set up and it is the most commonly used worldwide too.
- Botox is supplied as a vacuum dried powder and is stored in a refrigerator at or below 2C to 8C.
- The vial contains 100 units toxin that is diluted with NS to the desired final concentration.
- Once unsealed the toxin should be used within the next 4 hrs.
- Mixing of the solution should be done with least agitation & foaming.
The most important factor influencing the therapeutic window of any of the formulations of BTX is its ability to remain exclusively in the target tissue following injection.
Most adverse effects are caused by the systemic distribution or diffusion to the nearby noninjected muscle.
Practical points of injecting BOTOX
- Dose of BoNT-A
- Injecting volume
- Sites of BoNT-A injection
- Equipment needs
- Anesthesia/sedation
- Adverse events
- Repeat BoNT-A injection
Instruments for BoNT-A injections
- Rigid cystoscope – 14Fr, 17 Fr, for detrusor or urethral sphincter injection
- Flexible cystoscope – for urethral or detrusor injection
- Ureteroscope – 8 Fr, for pediatric injection of urethral sphincter or detrusor
- Injection cystoscopy – for suburothelial injection


Olympus 17F cystoscope and injection needle


Intravesical Injection Cystoscopy

Injection 23Fr cystoscope
A contingent self limiting needle can also be used

Injection apparatus

Flexible cystoscope and injection needle
Intravesical BoNT-A injections for NDO or IDO
- Local anesthesia (1%lidocaine 15min)/ IVGA
- Detrusor injection for NDO - 300U in 30ml, injecting 30 sites (Schurch B, et al, 2005
- Detrusor injection for IDO - 200U in 20ml, injecting 20 sites (Popat R, et al 2005)
- Detrusor injection for OAB - 100U in 30ml, injecting 30 sites (Werner M, et al, 2005)
- Suburothelial injection for IDO and NDO (Kuo HC, 2005) - 200U in 40ml, injecting 40 sites
- Trigonal injection? Reflux?
- Will BoNT-A leak from injecting site?
- How much perfusion area needed for a successful result

Intravesical BoNT-A injections
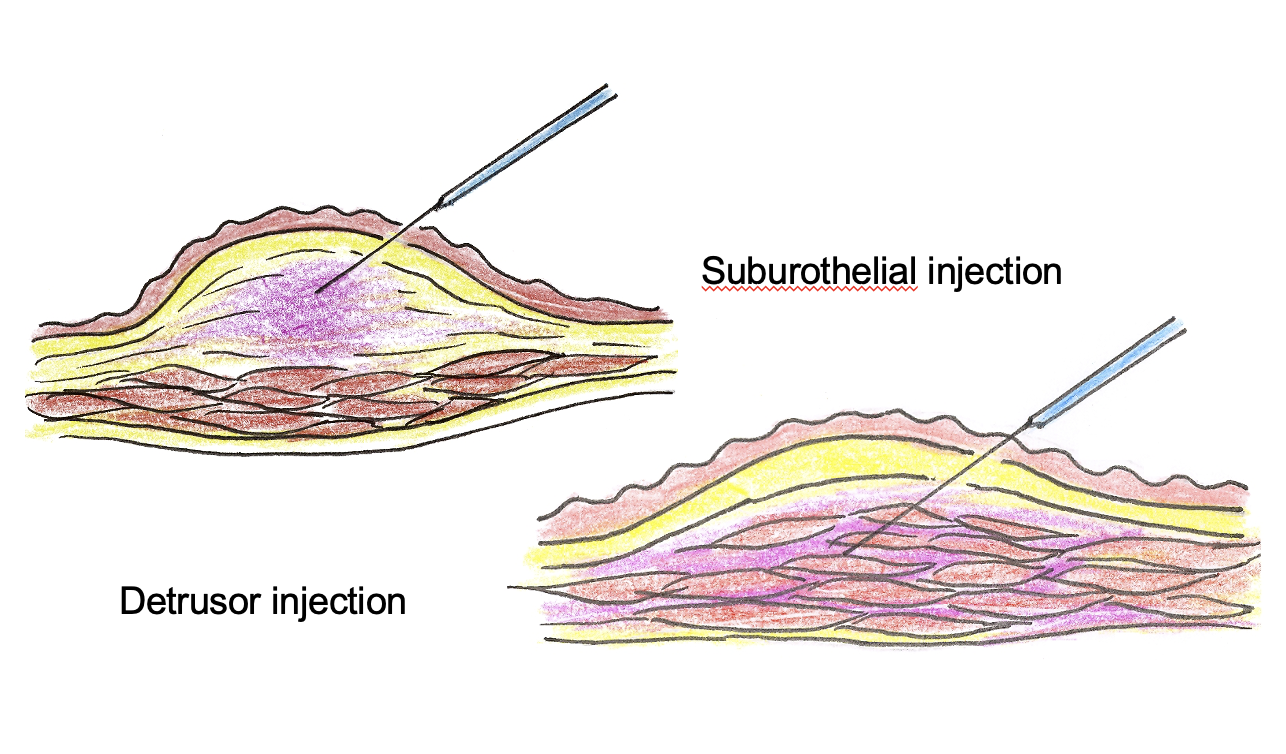
Detrusor vs suburothelial injection
Detrusor injections of BoNT-A for NDO
Adverse events related to BoNT-A injections
- Intravesical injections: difficult urination, transient retention, UTI, hematuria
- Urethral injections: stress incontinence, nocturnal overflow incontinence
- Prostatic injections: hematuria, acute prostatitis, transient retention
- General: weakness
- Transient erectile dysfunction
- Lethal dose 2000 to 3000 units in 70 kg human
